

In order to talk about the radius of an atom, we have to make an arbitrary decision about where the edge of the. Ionic Radius: A neutral atom changes to a cation by the loss of one or more electrons and to an anion by the gain of one or more electrons. Atomic Radii Trends in the Periodic Table. The term 'atomic radius' itself is problematic: it may be. The Vander Waals radius and covalent radius of chlorine atom are 1.80Å and 0.99Å respectively. With a metal, for example, the metallic atomic radius(r met)Half the distance between the nuclei of two adjacent metal atoms. The value assigned to the radius of a particular atom will always depend on the definition chosen for 'atomic radius', and different definitions are more appropriate for different situations. These electrons screen or shield the outer electrons from the nuclear charge. Atomic radius, and more generally the size of an atom, is not a precisely defined physical quantity, nor is it constant in all circumstances. Typically metallic and covalent radius is refered to as the atomic radius. This is because of the screening effect of the filled inner electron levels. The definition of atomic radius will vary slightly with the system under question. The greater attraction between the increased number of protons (increased nuclear charge) and electrons, pulls the electrons closer together, hence the smaller size.Īs you move down a group in the periodic table, the covalent radius increases. In other words, it is the distance from the center of.
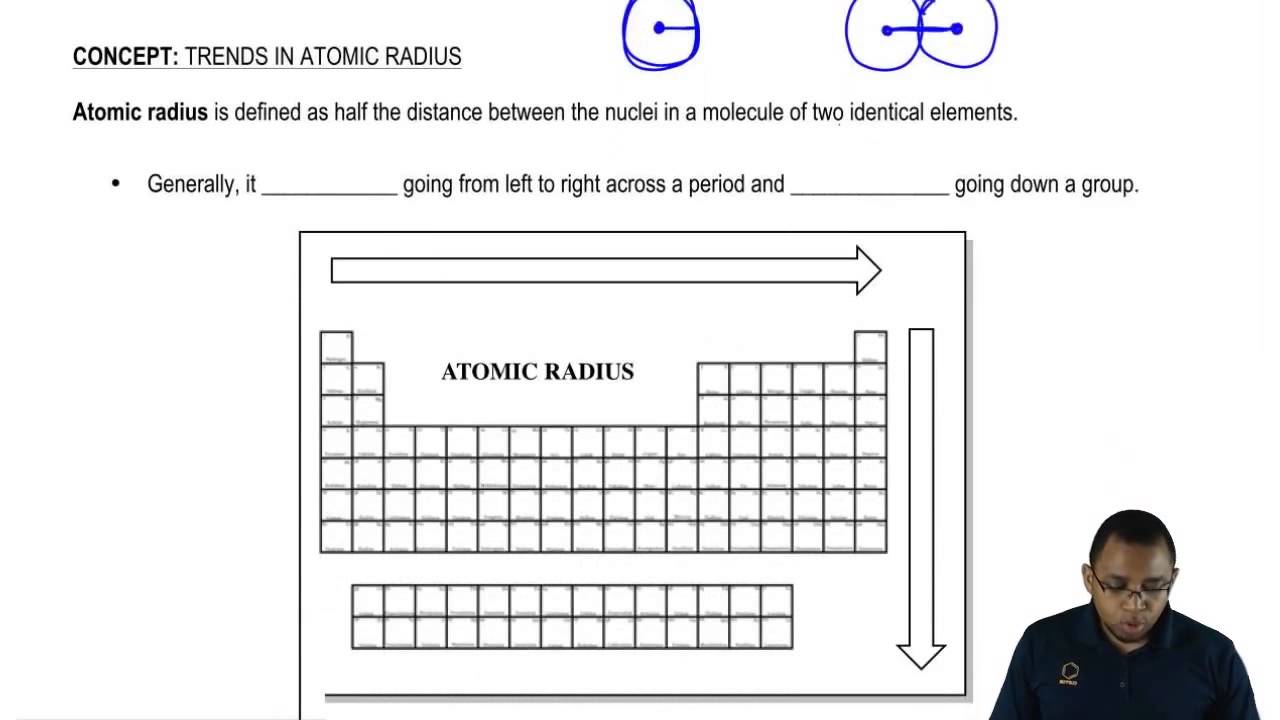
The covalent radius (a measure of how large individual atoms are) shows different trends if you are moving across a period or down a group.Ī comparison of the relative covalent radii of atoms is shown in the diagram below.Īcross a period from left to right, the covalent radius decreases.Īs you move from left to right across the periodic table, atoms have more electrons in their outer energy level and more protons in their nucleus. Atomic radius is the distance from the centre of the nucleus to the outermost shell containing electrons. Patterns and trends in the periodic tableĬhemists observe patterns in different properties of elements as they are arranged in the periodic table.


 0 kommentar(er)
0 kommentar(er)
